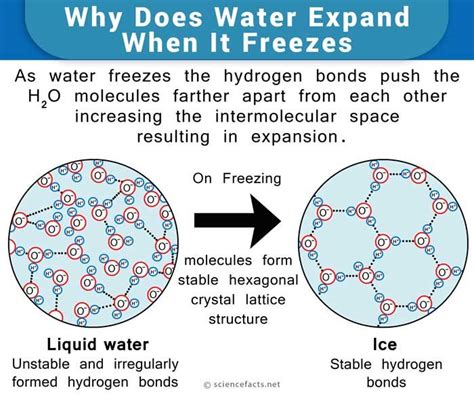
Water, ubiquitous yet enigmatic, continues to baffle scientists with its unique physical properties. To the layman, water might seem straightforward—clear, tasteless, life-sustaining. But beneath this simplicity lies a complex world of molecular dynamics that are crucial for life as we know it. One of the most interesting of these properties is water’s negative thermal expansion between 0 and 3.98°C. This phenomenon means that as water cools within this range, it expands rather than contracts, a property rarely seen in other substances. This anomaly is what causes ice to float, providing a thermal insulation layer on bodies of water and allowing life to thrive even in the depths of winter.
Water’s peculiar behavior raises questions that touch multiple scientific domains, from physics and chemistry to environmental science. The principle of negative thermal expansion contradicts our everyday understanding of how ‘heat rises.’ Instead of convection currents rising to the warmer surface, cold water sinks until it hits this critical point of 3.98°C. You can read more about negative thermal expansion here. This reversal in behavior sets the stage for many of water’s life-sustaining properties.
This unique property of water is indispensable for the survival of aquatic life during frigid conditions. As water cools and approaches the freezing point, the formation of ice at the surface creates a thermal barrier. This prevents the entire body of water from freezing solid, ensuring that the aquatic organisms below can survive. The density decrease of water as it solidifies into ice ensures that ice floats, creating a frozen layer on top while liquid water remains below at a stable 4°C. This concept is essential, not just for environmental scientists but for anyone curious about the underpinnings of ecological balance. If ice sank, aquatic ecosystems as we know them would not exist, highlighting water’s critical role in sustaining life.
What if ice didn’t float? The hypothesis that ice’s buoyancy is a ‘hacked’ feature of the universe brings a fascinating perspective. Imagine a universe where life as we know it hinges on such an intricate detail. As one commenter put it, this property may be proof of a designed universe, where other ‘design flaws’ necessitated a tweak to water. While this idea will surely provoke debate among both scientists and laypeople, it underlines an intriguing point: the fundamental properties of water are not just quirks but are vital for the complex processes that support life. To ponder further, check out this comprehensive article on ice formation.
Engineers and scientists are continually fascinated by water’s unique thermodynamics. One comment noted the irregular phenomenon of the Mpemba effect, where under certain conditions, hot water can freeze faster than cold water. This effect, though still a subject of much debate and study, adds another layer to our understanding of water’s unpredictable nature. If true, this phenomenon could be influenced by factors such as supercooling, convection currents, and even the properties of the container holding the water. To probe deeper into this curious effect, explore further here.
Finally, the complexities of water extend even to cellular biology, where its properties in microenvironments remain a topic of intensive research. Some fascinating studies suggest that water can maintain semi-solid states under particular conditions within cells, challenging our traditional view of biological processes. Cellular environments are not mere sacs of water; they are bustling, crowded places where molecules move with incredible speed and purpose. This perspective enhances our understanding of not just biological mechanisms but the fundamental principles of life itself. For an interesting visual portrayal of cellular processes, check out David Goodsell’s Molecular Landscapes here.
In every droplet of water, a world of scientific wonder exists. From its thermal anomalies to its role in sustaining ecosystems, water presents us with a conundrum: a seemingly simple substance that encapsulates the essence of life’s complexity. As science progresses, so too will our understanding of this indispensable molecule. Until then, we can marvel at how its simple existence layers our world with intricate beauty and ensures that life not only survives but thrives.

Leave a Reply